Nucleus® Implants
Cochlear provides a broad range of implants and electrodes to address your type of hearing loss, cochlea anatomy and surgeon preference.

What you will find on this page
- Learn how Cochlear™ Nucleus® Implants are designed
- Explore the Cochlear Nucleus Implant portfolio
- Discover the link between electrode design and hearing performance
- Learn about MRI considerations with Nucleus Implants
Cochlear’s Implant design philosophy
All our implants are designed to last a lifetime with performance and preservation of the cochlear structures in mind.* Our broad range of implants and electrodes allow your surgeon to choose the best one for you or your child's type of hearing loss, cochlea anatomy and the surgeon's preference. In addition, we design our implants with the future in mind by allowing for future advances in sound processor technology without the need for additional surgeries.
“I chose Cochlear because they have a very strong track record for making the newer technologies available to prior generation implants.”
- Oscar H. - Father of Nucleus® recipient
Nucleus® Profile™ Plus Implant
Industry-leading innovations in electrode design for optimal hearing performance
The anatomy of the cochlea can vary from person to person which is why we offer a range of electrode shapes and lengths. Your surgeon will decide on the best one to provide your best hearing performance. Unique features of Cochlear's electrode portfolio include:
-
22 active contacts for optimal hearing zone coverage1
-
The Hybrid L24 Electrode that is specifically designed and approved to preserve hearing
-
Perimodiolar electrodes that are designed to be inserted closest to the hearing nerve for your best hearing performance2,3
-
Best long-term implant reliability record in the industry4-6
-
Easier access to MRIs with magnets that can stay in place during scans7
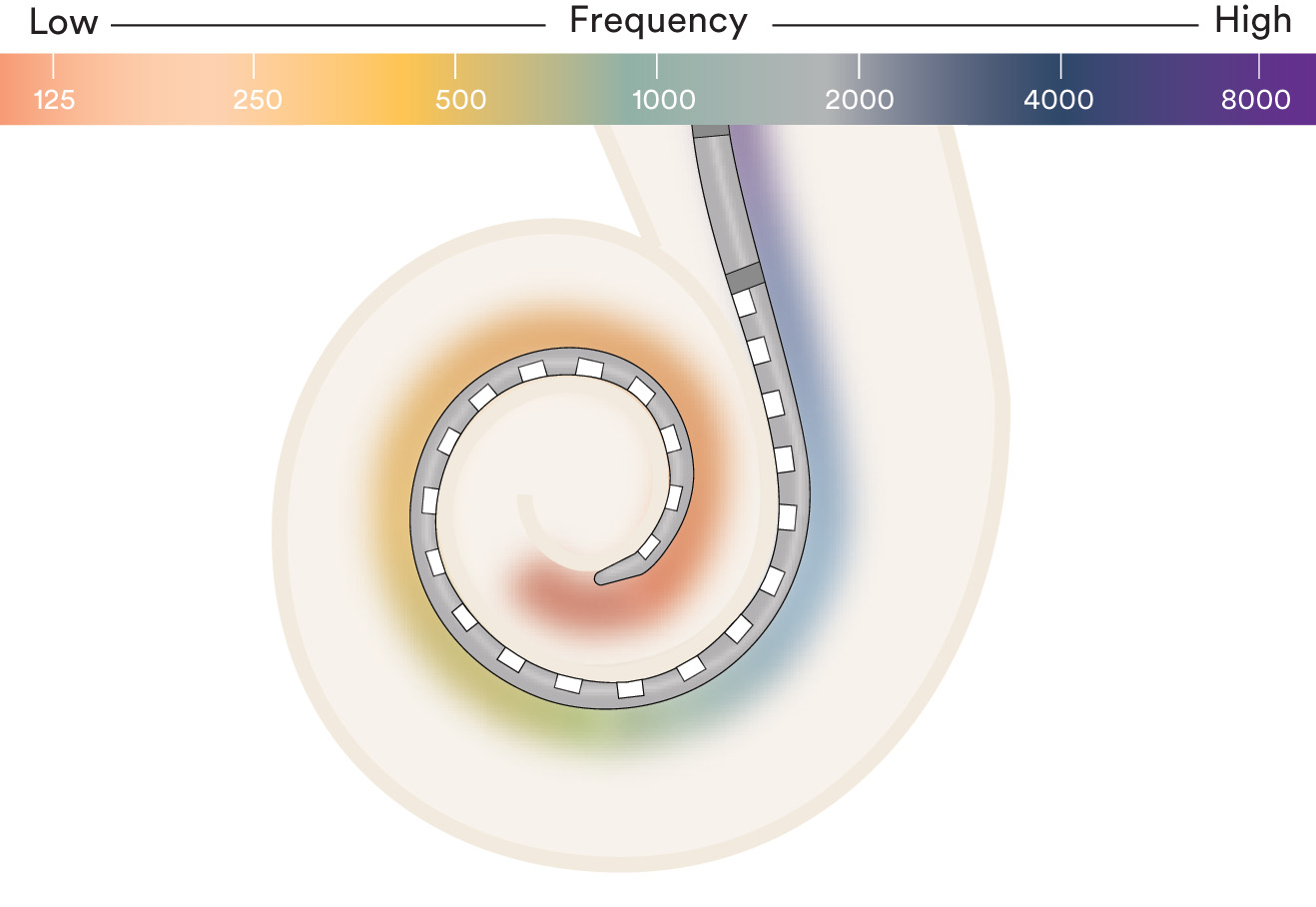
Electrode placement is critical to hearing performance
Cochlear's electrodes are intended to be placed where hearing nerve stimulation is most effective. In order to benefit from the full range of sound, the area closest to the hearing nerve tends to be stimulated.8,9 This area is known as the hearing zone.
Clinical research shows that deeper insertion beyond the hearing zone can be associated with deterioration in performance due to pitch confusion at the tip of the cochlea as well as damage to the delicate cochlear structures.9,10 Insertion depth along with the most active sequential contacts in the industry—22 electrodes1—help provide access to the full spectrum of sound and a richer hearing experience.
The Nucleus Profile Plus Implant provides easier access to MRIs
An MRI can be an important diagnostic tool for many therapeutic areas. Our newest generation Nucleus Implant - the Nucleus Profile™ Plus Implant - expands our innovative implant portfolio by offering access to MRIs at 1.5 and 3.0 Tesla without removing the magnet or requiring a head wrap.7
The Profile Plus Implant features a thin design that follows the natural curvature of the head for an improved aesthetic outcome and the potential of less time in surgery. In addition, it is built on the same platform as the industry's most reliable implant so you can feel confident that the implant will provide unparalled hearing peformance for years to come.4-6
It is important to recognize that there are alternative diagnostic imaging tools to replace an MRI, such as a CT scan. The most appropriate imaging tool should be discussed with your medical professional care team.
Interested in learning more?
If you are interested in learning more about the Nucleus System, we can send you a free informational guide today.
Disclaimer
*Contact Cochlear for details on specific product warranty information.
Please seek advice from your health professional about treatments for hearing loss. Outcomes may vary, and your health professional will advise you about the factors which could affect your outcome. Always read the instructions for use. Not all products are available in all countries. Please contact your local Cochlear representative for product information.
Views expressed by Cochlear recipients and hearing health providers are those of the individual. Consult your hearing health provider to determine if you are a candidate for Cochlear technology.
ACE, Advance Off-Stylet, AOS, AutoNRT, Autosensitivity, Beam, Button, CareYourWay, Carina, Cochlear, 科利耳, コクレア, Cochlear SoftWear, Codacs, ConnectYourWay, Contour, Contour Advance, Custom Sound, ESPrit, Freedom, Hear now. And always, HearYourWay, Hugfit, Hybrid, Invisible Hearing, Kanso, MET, MicroDrive, MP3000, myCochlear, mySmartSound, NRT, Nucleus, Off-Stylet, Slimline, SmartSound, Softip, SPrint, True Wireless, the elliptical logo, WearYourWay and Whisper are either trademarks or registered trademarks of Cochlear Limited. Ardium, Baha, Baha SoftWear, BCDrive, DermaLock, EveryWear, Vistafix, and WindShield are either trademarks or registered trademarks of Cochlear Bone Anchored Solutions AB.
References:
- Technical Specifications Cochlear™ Nucleus® Profile™ Plus (CI612). Data on file.
- Holden LK, Finley CC, Firszt JB, Holden TA, Brenner C, Potts LG, et al. Factors affecting open-set word recognition in adults with cochlear implants. Ear Hear.2013 May-Jun;34(3):342-60.
- Esquia Medina, GN., Borel, S., Nguyen, Y., Ambert-Dahan, E., Ferrary, E., Sterkers, O., Bozorg Grayeli, A. Is Electrode-Modiolus Distance a Prognostic Factor for Hearing Performances after Cochlear Implant Surgery. Audiol Neurotol. 2013;18:406-413. DOI: 10.1159/000354115.
- Cochlear Nucleus Implant Reliability Report. Volume 17 | December 2018. D1593476. Cochlear Ltd; 2019.
- Hearing Implant Reliability Reporting | MEDEL [Internet]. MedEl.com. 2018 [cited 24 Oct 2018]. Available from: https://www.medel.com/us/reliabilityreporting.
- 2018 Global Reliability Report. 027-N025-02 Rev B. Advanced Bionics AG and Affiliates.; 2018.
- MRI Guidelines D774756.
- Stakhovskaya O., Sridhar, D., Bonham, B.H., Leake, P.A. Frequency map for the human cochlear spiral ganglion: Implications for Cochlear Implants. JARO,2007; 8(2): 220-233.
- Ariyasu, L., Galey, FR., Hilsinger, RJR., Byl, FM. Computer-generated three dimensional reconstruction of the cochlea. Otolaryngology - Head and Neck Surg 1989; 100(2): 87.
- Gani M, Valentini G, Sigrist A, Kos MI, Boex C. Implications of deep electrode insertion on cochlear implant fitting. J Assoc Res Otolaryngol. 2007 Mar; 8(1):69-83.