Osia® Implant
The first and only active bone conduction system to enable MRI at 3.0 T.
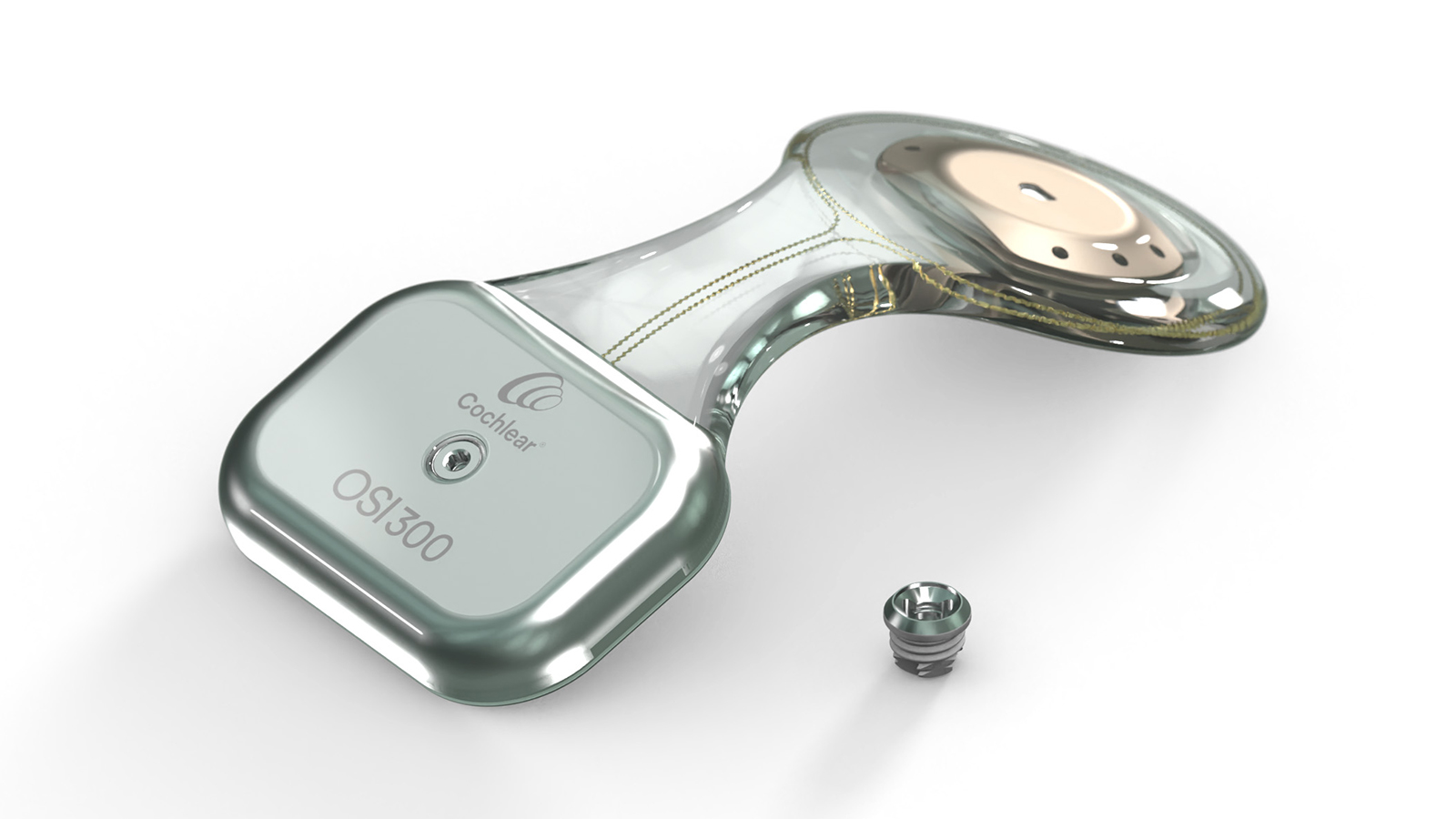
The Osia OSI300 implant
Like no other hearing implant, the Osia System uses a piezoelectric transducer to send sound directly to the cochlea. The Piezo Power™ transducer is made of piezoelectric layers that expand and contract to send vibrations through to the cochlea. The piezoelectric effect is the ability of certain materials to generate an electrical charge from mechanical stress, or in reverse, to generate vibrations from an electrical charge.
The advantages of the Piezo Power transducer include the ability to deliver high-frequency power in a slim design and compatibility for MRI as there is no magnetic material and no performance degradation after MRI exposure, as there is nothing to be demagnetized as in an electromagnetic transducer.1-3
With the OSI300 Implant, the Cochlear Osia System becomes industry’s first and only active bone conduction system to enable MRI at 3.0 T, allowing patients to undergo MRI scans with implant magnet in place, without the need of removing the magnet or the use of a headwrap. This patient benefit is made possible by combining the unique Piezo Power™ technology in the transducer, which does not contain magnetic material, and the next generation implant magnet technology for 3.0 T.
The OSI300 uses a diametric magnet that sits within a casing and rotates to align with the magnetic field of the MRI machine.
The OSI300 Implant is only compatible with the Osia 2(I) Sound Processor.
Disclaimer
This material is intended for health professionals. If you are a consumer, please seek advice from your health professional about treatments for hearing loss. Outcomes may vary, and your health professional will advise you about the factors which could affect your outcome. Always read the instructions for use. Not all products are available in all countries. Please contact your local Cochlear representative for product information.
For a full list of Cochlear’s trademarks, please visit our Terms of Use page.
References
- Osia System R5 Datasheet. Cochlear Limited, Sweden. 2023; D1991788.
- Osia OSI200 Implant Technical Brief. Cochlear Limited, Sweden. 2020; D1602089.
- Goh J. OSI200 Implant MRI Safety Verification Report. D1439962. Cochlear Bone Anchored Solutions AB, Sweden 2019.







