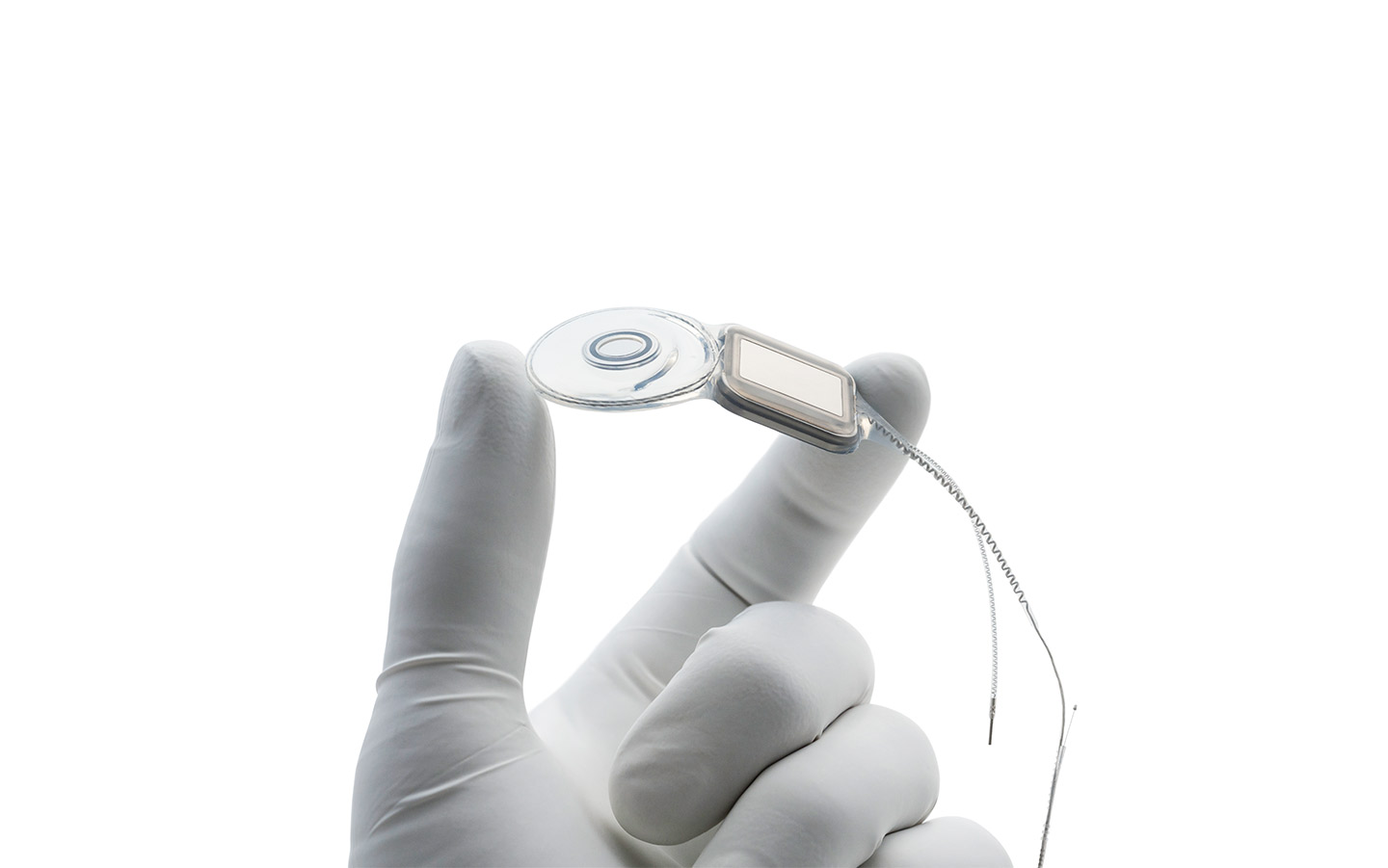
Nucleus® Implants
Our Cochlear Implants support a lifetime of hearing. Engineered with precision, they are designed to fit the natural shape of the inner ear, preserving the long-term health of the cochlea.

What you'll find on this page
- How we design Cochlear™ Nucleus® Implants.
- The Cochlear Nucleus Implant portfolio.
- The link between electrode design and hearing performance.
Cochlear's implant design philosophy
- Designed to support a lifetime of hearing performance.*
- Electrodes designed to fit the natural shape of the inner ear.1
- Support advances in sound processor technology.
World's thinnest cochlear implants
The Nucleus® Profile™ and Profile Plus Series Implants are the thinnest in the world.2
Designed to fit the natural shape of your inner ear, sitting closer to the hearing nerve for better hearing outcomes.3-5
Both Profile Plus and Profile Series Implants also have a high impact resistance — up to 2.5 joules — which meets the European standards for impact testing.
Industry-leading design for hearing performance
The anatomy of the cochlea varies from person to person. That's why we offer a range of electrode shapes and lengths. Your surgeon will decide which one is best for you.
Unique features of Cochlear's electrodes and implants include:
- 22 active contacts for precise frequency coverage.
- The world's thinnest full-length electrode.6
- Electrodes which sit closer to the hearing nerve for better hearing performance.3-5
- Most reliable cochlear implants over time.**,7

Disclaimer
Please seek advice from your health professional about treatments for hearing loss. Outcomes may vary, and your health professional will advise you about the factors which could affect your outcome. Always follow the directions for use. Not all products are available in all countries. Please contact your local Cochlear representative for product information.
For a full list of Cochlear’s trademarks, please visit our Terms of Use page.
* “A lifetime of hearing performance” and similar phrases should not be understood as claims relating to the expected life, reliability, quality or performance of Cochlear’s products.
** Based on comparable implant generations released by Cochlear, MED-EL and Advanced Bionics using each manufacturer’s first published CSP data at 7 and 15 years.
References
- Shaul C, Dragovic AS, Stringer AK, O’Leary SJ, Briggs RJ. Scalar localisation of peri-modiolar electrodes and speech perception outcomes. J Laryngol Otol. 2018;132:1000–6.
- Cochlear Limited. D1655096. Competitive Comparison of Implant Rec/Stim Thickness. 2019, August.
- Ramos, Shaul and Holden: Shaul C, Dragovic AS, Stringer AK, O’Leary SJ, Briggs RJ. Scalar localisation of peri-modiolar electrodes and speech perception outcomes. J Laryngol Otol. 2018;132:1000–6.
- Holden LK, Finley CC, Firszt JB, Holden TA, Brenner C, Potts LG, et al. Factors affecting open-set word recognition in adults with cochlear implants. Ear Hear 2013; 34: 342-60.
- Ramos de Miguel A, Argudo AA, Borkoski Barreiro SA, Falcon Gonzalez JC, Ramos Macias A. Imaging evaluation of electrode placement and effect on electrode discrimination on different cochlear implant electrode arrays. Eur Arch Otorhinolaryngol. 2018 Jun;275(6):1385-1394.
- Cochlear Limited. D1655106. Competitive Comparison of Implant Intracochlear Electrode Thickness. 2021, September.
- Cochlear Limited. D2325238 Cochlear Nucleus Reliability Report Volume 23 December 2024.