Vistafix® system
Learn about the individual components that make up Cochlear's Vistafix system implant and abutments.
The Vistafix System is no longer available for purchase and will be retired on May 18, 2025. Supporting System documentation can continue to be found during this time under Vistafix Surgical Guides. Prosthetic and lab components will continue to be available.
How the Vistafix system works
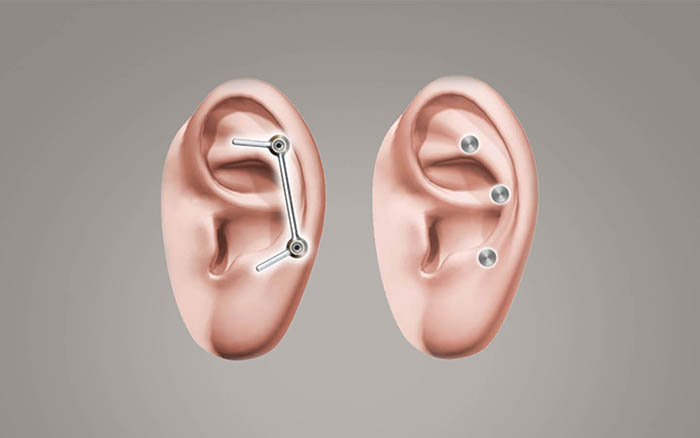
The Vistafix system consists of osseointegrated implants, abutments, and a retention mechanism for the prosthesis.
There are two types of prosthetic retention mechanisms commonly used: bar & clip, or magnet retained systems. Either option enables patients to attach or remove the prosthesis themselves, with minimal effort.
- The Vistafix implants are inserted into the bone.
- After an osseointegration period of typically 3-6 months, the surgeon will attach the abutments to the implants.
- After the abutments are placed, the anaplastologist will make impressions of the site, and craft the prosthetic. In auricular cases, the anaplastologist will take care to create symmetry with the opposite side.
- Final adjustments are made during the prosthetic fitting.
Implant
VXI300 Implant
The VXI300 Implant is the strong foundation of Vistafix Systems. With a TiOblast™ surface it provides long term stability compared with the previous generation of machined surface implant. The Vistafix 3 Implant has the same design as the Baha 3 Implant, which has been clinically demonstrated to provide significantly improved implant stability.1-3
Abutments
VXA300 Abutments
The Vistafix 3 System features four different abutment heights, enabling the height of the retention structure to be adapted according to the nature of the defect and the varying clinical needs and unique circumstances of each patient. The abutment-to-implant connections, combined with the abutment shape of the Vistafix 3 System, have been designed to reduce soft tissue complications. Fewer soft tissue complications can reduce the number of outpatient visits, which is beneficial to both patients and professionals.
Disclaimer
This material is intended for health professionals. If you are a consumer, please seek advice from your health professional about treatments for hearing loss. Outcomes may vary, and your health professional will advise you about the factors which could affect your outcome. Always read the instructions for use. Not all products are available in all countries. Please contact your local Cochlear representative for product information.
For a full list of Cochlear’s trademarks, please visit our Terms of Use page.
References
- Dun CA, de Wolf MJ, Hol MK, Wigren S, Eeg-Olofsson M, Green K, Karlsmo A, Flynn MC, Stalfors J, Rothera M, Mylanus EA, Cremers CW. Stability, survival, and tolerability of a novel Baha implant system: six-month data from a multicenter clinical investigation. Otology & Neurotology 2011 Aug; 32(6):1001-7.
- Gottlow J, Sennerby L, Rosengren A, Flynn M. An experimental evaluation of a new craniofacial implant using the rabbit tibia model. Part I. Histological findings. Otology & Neurotology 2010;31(5):832-9.
- Sennerby L, Gottlow J, Rosengren A, Flynn M. An experimental evaluation of a new craniofacial implant using the rabbit tibia model. Part II. Biomechanical findings. Otology & Neurotology 2010;31(5):840-5.