Cochlear Implant Candidacy Information

Cochlear Implant Candidacy Criteria
Adults
- Individuals 18 years of age or older
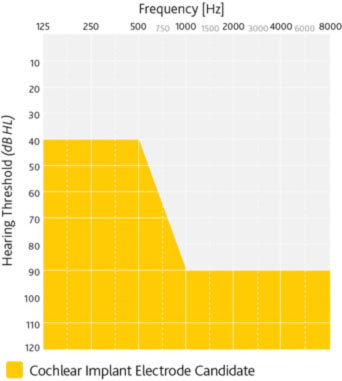
- Moderate to profound sensorineural hearing loss in both ears
- Limited benefit from amplification defined by preoperative test scores of ≤ 50% sentence recognition in the ear to be implanted and ≤60% in the opposite ear or binaurally1
Children (2-17 Years)
- Severe to profound sensorineural hearing loss in both ears
- Limited benefit from binaural amplification
- Multisyllabic Lexical Neighborhood Test (MLNT) or Lexical Neighborhood Test (LNT) scores ≤ 30%
Children (9-24 Months)*^
- Profound sensorineural hearing loss in both ears
- Limited benefit from binaural amplification
Important Considerations:
- Remember that many individuals with residual low frequency hearing are CI candidates and can benefit from CI.
- When appropriate, discuss use of acoustic hearing: Does your patient have a passion for music? Work in noisy or reverberant environments? What are lifestyle demands?
- Determine motivation to experiment with hearing aids, in both the implanted and/or contralateral ears.
- Discuss the opportunity to use acoustic hearing to manage expectations and rehabilitation options, when appropriate.
Nucleus Hybrid™ Implant Candidacy Criteria
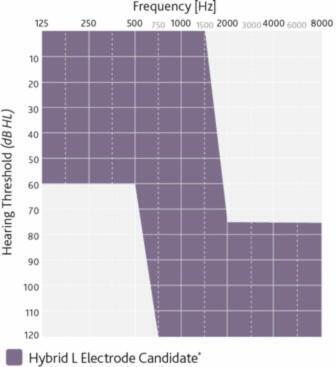
The Nucleus Hybrid System is indicated for unilateral use in patients aged 18 years and older who have residual low frequency hearing sensitivity and severe to profound high-frequency sensorineural hearing loss, and who obtain limited benefit from appropriately fitted bilateral hearing aids.
- Typical preoperative hearing of candidates ranges from normal to moderate hearing loss in the low frequencies (thresholds better than 60 dB HL up to and including 500 Hz) and
- Severe to profound mid to high-frequency hearing loss (threshold average of 2000, 3000, and 4000 Hz ≥75 dB HL) in the ear to be implanted.
- For individuals with post-surgical low frequency hearing, the sound processor combines acoustic amplification for the low frequencies with electric stimulation for the high frequencies.
- The CNC word recognition score criteria are between 10% and 60%, inclusively, in the ear to be implanted in the preoperative aided condition.
- The contralateral ear’s CNC score criteria are equal to or better than that of the ear to be implanted, but not better than 80% correct.
- Moderately severe to profound mid- to high frequency hearing loss (threshold average of 2000, 3000, and 4000 Hz ≥ 60 dB HL) in the contralateral ear.
Disclaimer
This material is intended for health professionals. If you are a consumer, please seek advice from your health professional about treatments for hearing loss. Outcomes may vary, and your health professional will advise you about the factors which could affect your outcome. Always read the instructions for use. Not all products are available in all countries. Please contact your local Cochlear representative for product information.
For a full list of Cochlear’s trademarks, please visit our Terms of Use page.
*In Canada, the Cochlear Nucleus Profile Plus (CI600) Series and Profile (CI500) Series Implant Systems are intended for use in children 9 to 24 months of age who have bilateral profound sensorineural deafness and demonstrate limited benefit from appropriate binaural hearing aids. Cochlear Nucleus Freedom Series (CI24RE (CA) and (ST), and CI422) Implant System is intended for use in children 12 to 24 months of age who have bilateral profound sensorineural deafness and demonstrate limited benefit from appropriate binaural hearing aids. Children 2 years of age or older may demonstrate severe to profound hearing loss bilaterally for both series of implants.
^To reduce the risk of anesthetic-related adverse events, a pediatric anesthesiologist should be present during surgery for infants implanted under 12 months of age.
For complete guidelines on Cochlear™ Nucleus® indications please refer to the appropriate Cochlear™ Nucleus® Physician’s Guide.